Spontaneous onset of cellular markers of inflammation and genome instability during aging in the immune niche of the naturally short-lived turquoise killifish (Nothobranchius furzeri) (Research Article)
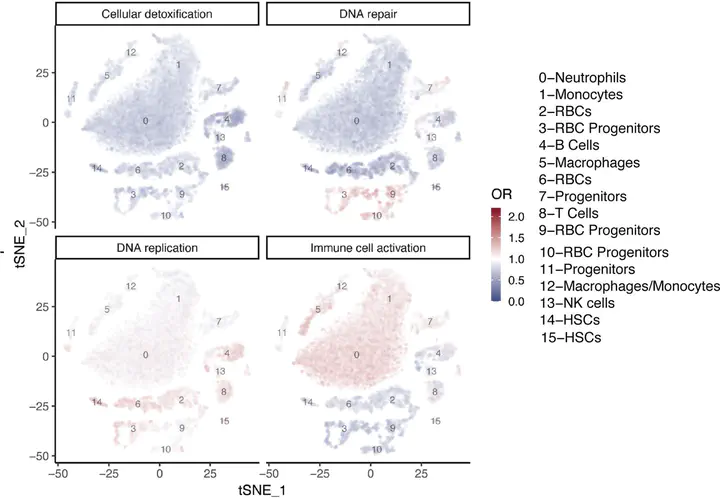 Figure 5 - bioRxiv J
Figure 5 - bioRxiv J
Abstract
Turquoise killifish (Nothobranchius furzeri) are naturally short-lived vertebrates, recapitulating several aspects of human aging, including protein aggregation, telomere shortening, cellular senescence, and declined antibody diversity. The mechanistic causes of systemic aging in killifish are still poorly understood. Here we ask whether killifish undergo significant age-dependent changes in the main hematopoietic organ, which together could contribute to systemic aging. To characterize immune aging in killifish, we employed single-cell RNA sequencing, proteomics, cytometry and a functional in vitro assay. Our data indicate how old killifish display increased inflammatory markers, and while immune cells from adult killifish display increased markers of proliferation and replication-independent DNA repair in progenitor-like cell clusters, progenitors from old killifish display extensive markers of DNA double-strand breaks. In less than 10 weeks, killifish undergo several dramatic spontaneous aging-related changes in the immune niche, which could be functionally linked with its extensive systemic aging and serve as targets for anti-aging interventions.